AREA TEMATICA: Ibd
Authors:
Fabiana Castiglione1, Alessia Dalila Guarino1, Biagio Pinchera2, Giuseppe Spadaro3, Emanuela Zappulo2, Giulio Calabrese1, Antonio Rispo1, Anna Testa1, Olga Maria Nardone4, Caterina Mucherino5, Agnese Miranda6, Giuliana Vespere7, Nicola Imperatore8, Pietro Capone9, Marta Patturelli10, Antonietta Gerarda Gravina11, Lucienne Pellegrini12, Ivan Gentile2
Affiliations:
- Gastroenterology, University of Naples Federico II
- Department of Clinical Medicine and Surgery – Infectious Diseases, Università di Napoli “Federico II,” Naples, Italy
- Department of Translational Medical Sciences, University of Naples Federico II
- Department of Public Health, University Federico II of Naples
- UOC Gastroenterologia ed Endoscopia Digestiva, AORN Sant’Anna e San Sebastiano, Caserta
- U.O.C. Gastroenterologia ed Endoscopia digestiva, Monaldi, Ospedale dei Colli, Napoli
- UOC Gastoenterologia, Ospedale del Mare, Napoli
- Gastroenterologia ed Endoscopia digestiva, Santa Maria delle Grazie, Pozzuoli (NA)
- U.O.C. Gastroenterologia ed Endoscopia Digestiva, Maresca, Torre del Greco (NA)
- UOC Gastroenterologia, AORN Cardarelli, Napoli
- Università degli Studi della Campania – Vanvitelli
- UOC Gastroenterologia, San Giovanni di Dio e Ruggi d’Aragona (SA)
Background and aims:
Patients with inflammatory bowel disease (IBD) have a higher risk of varicella zoster virus infection (VZI) than the general population,largely due to immunosuppressive or biologic therapies[1].Preventing viral reactivation is a relevant clinical goal[2].The recombinant zoster vaccine(RZV, Shingrix)has shown high efficacy and good tolerability in the general population,butdata in IBD are limited[1,2].Real-world evidence on its effectiveness and safety profile in this setting is needed to support vaccination strategies and guide clinical practice[2,3].The objective of the study was to assess the effectiveness and safety of the RZV in IBD.
Methods:
From March 2023 to September2024,consecutive IBD patients who received the RZV Shingriximmediately before starting or with ongoing biologics were prospectively enrolled across 9 tertiary IBD centers.Clinical data were collected at baseline.Effectiveness was assessed clinically and defined as the absence of VZI orHZ reactivation during the observation period.Safety outcomes included the occurrence of vaccine-related adverse events(AEs)—such as fever, injection site pain, arthralgia, and others—systematically recorded during the follow-up after each vaccine dose.
Results:
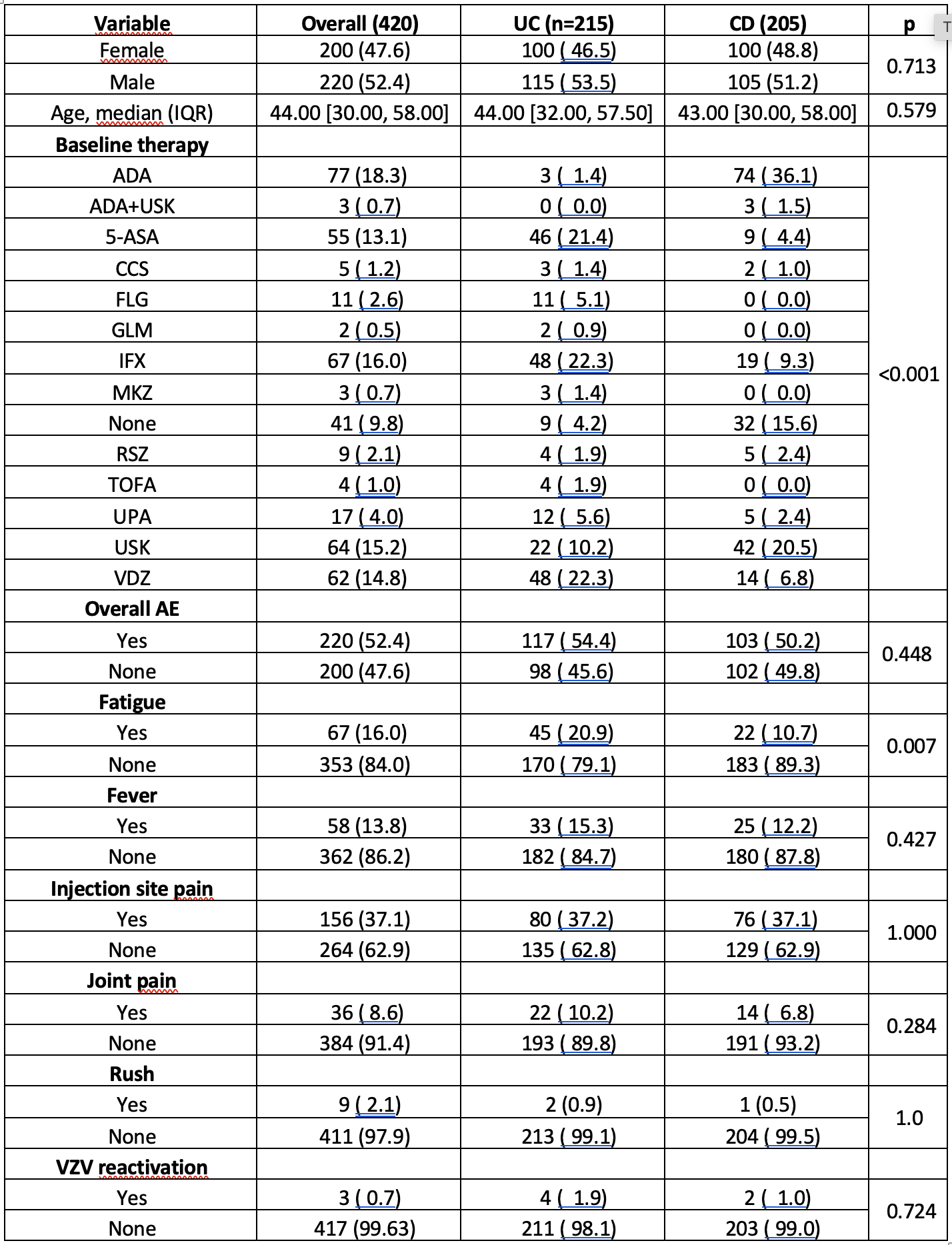
A total of 420 IBD patients were included,comprising 215(51.2%)with UC and 205(48.8%) with CD, with a median age of 44 years(Table 1).Most patients(95.8%)completed the VZV vaccination cycle.Biologic therapy was ongoing in 319(75.9%)patients,more frequently in CDthan UC(p< 0.001).During a mean follow-up of 11.0 ± 1.2 months,VZV reactivation occurred in only 0.7% of patients, confirming a high clinical effectiveness of RZV in IBD.Overall,AEs were reported in 52.4% of cases,with the most common being arm pain(37.1%),followed by asthenia(16.0%)and fever(13.8%).No significant differences between UC andCD(54.4% vs 50.2%,p=0.448),as for gender(p=0.96),were found.AE occurrence was significantly associated with ongoing biologic therapy(56.9% vs 29.3% without,p=0.009)and lower age (median42 vs 45.5years,p=0.047).However,analyzing therapies by specific mechanism of action, including JAKi,no class of drug was associated with a higher AE risk compared to others.Logistic regression confirmed only biologics as an independent risk factor(OR1.84;95%;p=0.009).In the subgroup analysis comparing biologics to conventional or no therapy,only asthenia and joint pain were significantly more common in patients on biologics(p<0.001 and p=0.04, respectively).No serious AEs were reported.
Conclusions:
In this prospective cohort,RZV demonstrated a favorable safety profile in IBD patients,with only mild and self-limiting adverse events,more frequently observed in those receiving biologics. Notably, the very low rate of VZV reactivation(0.7%)over a mean follow-up of 11months confirms the high effectiveness of RZV in this high-risk population.These findings support the integration of RZV into preventive strategies in IBD,particularly before or during immunosuppressive therapy.
Bibliography:
- Kim DH, Kang SB. Herpes zoster infection in patients with inflammatory bowel disease. Korean J Intern Med. 2025 May; 40 (3): 347-356. doi: 10.3904/kjim.2024.342.
- Din S, Selinger CP, Black CJ, Ford AC. Systematic review with network meta-analysis: Risk of Herpes zoster with biological therapies and small molecules in inflammatory bowel disease. Aliment Pharmacol Ther. 2023 Mar; 57 (6): 666-675.
- Nugent Z, Singh H, Targownik LE, Bernstein CN. Herpes Zoster Infection and Herpes Zoster Vaccination in a Population-Based Sample of Persons With IBD: Is There Still an Unmet Need? Inflamm Bowel Dis. 2019 Feb 21; 25 (3): 532-540.
Table 1
VALUTA ABSTRACT
Vota ogni categoria
| Originality | |
| Clarity | |
| Rationale | |
| Objectives | |
| Endpoint measures | |
| Statistics | |
| Results | |
| Relevance | |
|
Media
|
|
|
|


