AREA TEMATICA: Ibd
Authors:
M. Murgiano1, F. Di Vincenzo1,2, P. Puca1,2, A. Del Gaudio1,2, S. Parello1, F. Cascella1, F. Fiaccavento1, A. Gasbarrini1,2, L.R. Lopetuso2,3, L. Laterza1,2, F. Scaldaferri1,2
Affiliations:
- Dipartimento di Medicina e Chirurgia Traslazionale, Università Cattolica del Sacro Cuore, 00168 Rome, Italy
- Medicina Interna e Gastroenterologia, CEMAD Centro Malattie dell’Apparato Digerente, Dipartimento di Scienze Mediche e Chirurgiche, Fondazione Policlinico Universitario Gemelli IRCCS, 00168 Rome, Italy
- Dipartimento di Scienze della Vita, della Salute e delle Professioni Sanitarie, Università degli Studi Link, 00165 Rome, Italy
Background and aims:
Subcutaneous infliximab (IFX-SC) is a novel formulation of the anti-TNF monoclonal antibody infliximab, approved for the treatment of inflammatory bowel diseases (IBD). Following standard intravenous induction (typically at weeks 0 and 2), maintenance treatment is administered as a fixed 120 mg subcutaneous injection every two weeks. IFX-SC enables self-administration, potentially enhancing treatment adherence and reducing healthcare utilization. In clinical practice, dose optimization—through interval shortening or dose adjustment—may be required in selected patients. In cases of suboptimal response or tolerability issues, switching back to intravenous infliximab remains a viable option. Despite promising pharmacokinetic and immunogenicity profiles, long-term real-world data on the effectiveness and safety of IFX-SC remain limited.
Methods:
From November 2020 to June 2025, 202 IBD patients treated with subcutaneous IFX (IFX-SC), were retrospectively enrolled. All patients who switched from intravenous to IFX-SC—either during induction or after at least 6 months of intravenous therapy—were included, provided they had a minimum follow-up of 6 months after the switch. Clinical characteristics, blood and fecal biomarkers, disease activity scores, steroid use, extraintestinal manifestations, and major adverse outcomes were collected. The primary endpoint was the persistence of steroid-free clinical remission at 6 to 12 months. Secondary endpoints included persistence of biochemical remission (defined as fecal calprotectin <250 µg/g and CRP ≤5 mg/L) and endoscopic remission (Mayo score = 0 for UC; SES-CD <2 or Rutgeerts i0 for CD) at 6–12 months, as well as clinical, biochemical, and endoscopic remission beyond 12 months. Additional outcomes were the occurrence of adverse events and the need for treatment optimization or reversion to intravenous administration.
Results:
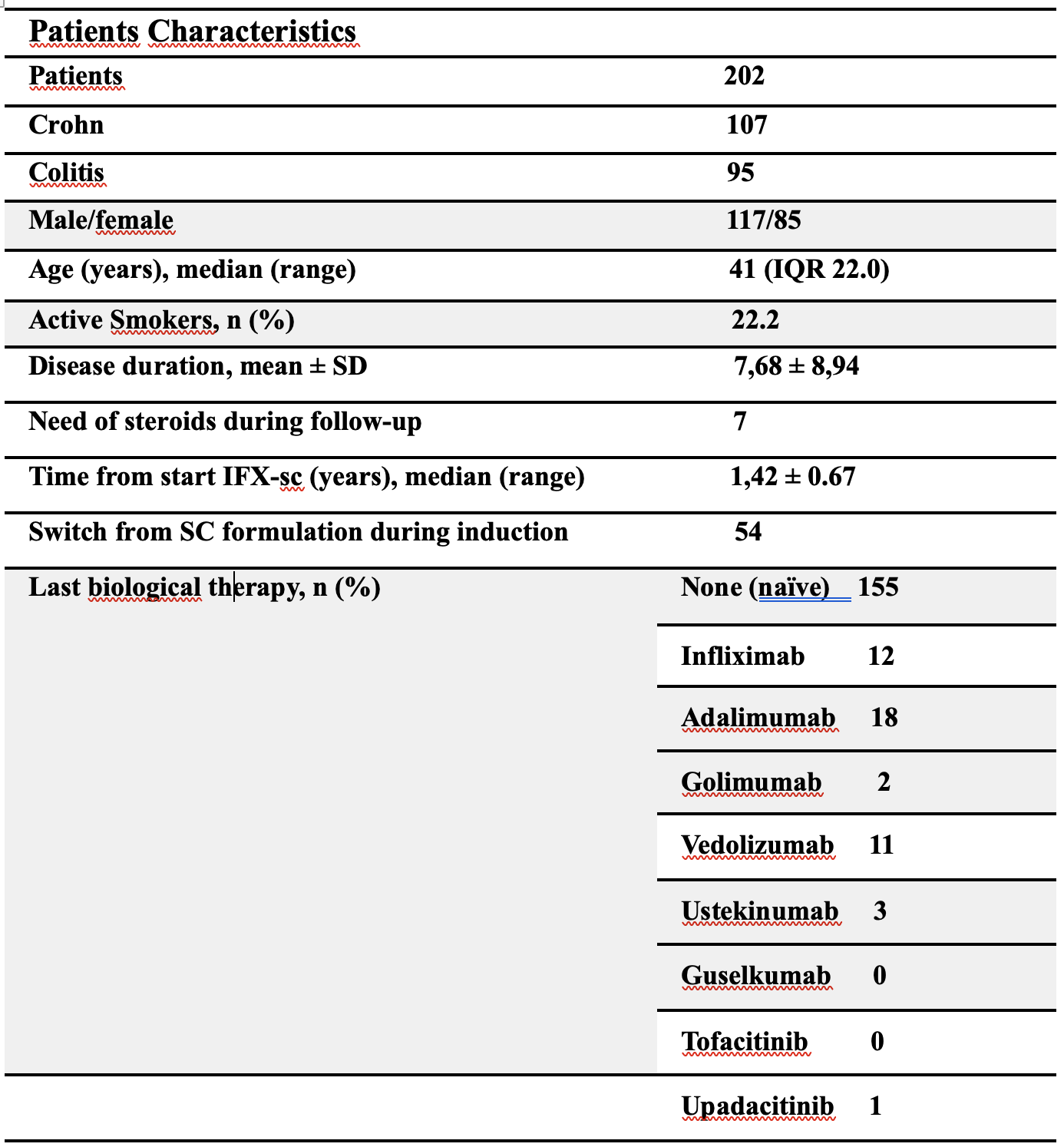
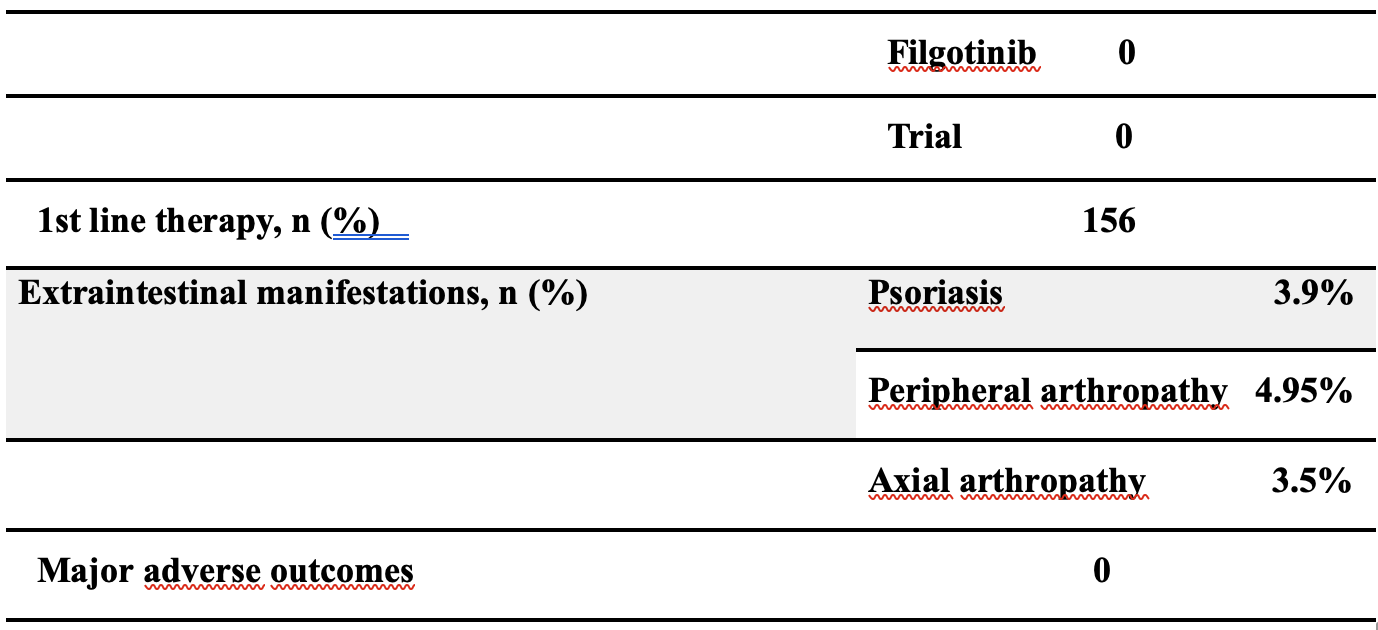
A total of 202 IBD patients (95 with UC and 107 with CD) were included. Of these, 156 (77.2%) received Infliximab as first-line. 54 (26.7%) patients initiated subcutaneous infliximab (IFX-SC) during induction, while 148 (73.2%) switched to IFX-SC after at least 6 months of intravenous infliximab (maintenance group). In CD patients, steroid-free clinical remission was maintained in 95% at 6 months, 80% at 12 months, and 65% beyond 12 months. In UC patients, corresponding rates were 77%, 67%, and 50%, respectively. During follow-up, 7 patients (3.4%) required corticosteroid therapy for disease flares. Overall, 13 patients discontinued IFX-SC: 3 within the first 6 months (2 UC, 1 CD) and 10 from the maintenance group (5 UC, 5 CD). Six patients (3 UC, 3 CD) reverted to intravenous infliximab, mostly within the first 6 months, due to loss of response or patient preference. No serious adverse events or IBD-related complications were reported during the observation period.
Conclusions:
In this real-world cohort of IBD patients, subcutaneous infliximab (IFX-SC) demonstrated long-term steroid-free clinical remission, particularly in Crohn’s disease, with favorable tolerability and a low discontinuation rate. The switch from intravenous to subcutaneous formulation was generally safe and effective, both when initiated during induction and in maintenance. These findings support the long-term use of IFX-SC as a viable and well-tolerated alternative for selected IBD patients in clinical practice.
Table 1
VALUTA ABSTRACT
Vota ogni categoria
| Originality | |
| Clarity | |
| Rationale | |
| Objectives | |
| Endpoint measures | |
| Statistics | |
| Results | |
| Relevance | |
|
Media
|
|
|
|


