AREA TEMATICA: General gastro
Authors:
Pierfrancesco Visaggi, Gaia Cairoli, Alberto Barchi, Edoardo Vespa, Mauro Mitilini, Federico Testi, Lorenzo Bini, Isabella Dulmin, Edoardo Vincenzo Savarino and Nicola de Bortoli
Background and aims:
Budesonide orodispersible tablet (BOT) is the only approved topical steroid available in Europe for the induction and maintenance of remission in patients with eosinophilic esophagitis (EoE). Available randomized controlled trials have demonstrated that, following induction of histological remission with BOT 1mg twice daily (bid), patients can be maintained in remission with BOT 0.5mg bid. It is currently unknown whether a dose of 1mg once daily (od) has comparable efficacy to 0.5mg bid.
Methods:
In this prospective study conducted at two tertiary referral centers for EoE, we enrolled consecutive patients with EoE undergoing clinical, endoscopic, and histologic re-assessment while on maintenance treatment for EoE with BOT 0.5mg bid or BOT 1mg od. All included patients had achieved histological remission (i.e., <15 eosinophils/high-power field, eos/HPF) following induction of remission with BOT 1mg bid. At the end of the induction of remission period (T0), all patients were assessed using the dysphagia symptom questionnaire (DSQ), the EoE endoscopic reference score (EREFS), and peak eosinophil counts (PEC). At re-assessment while on maintenance treatment (T1), all patients underwent 1) assessment of dysphagia and adaptive behaviors with the DSQ and the Pisa EoE Adaptation Questionnaire v1.0 (PiEAQ). Clinical remission was defined as DSQ not increasing >30% compared to T0. 2) upper endoscopy (EGDS) with assessment of EREFS score and at least six esophageal biopsies, and 3) histological assessment using the EoE Histology Scoring System (EoEHSS). In addition, a comprehensive global assessment of clinical, endoscopic, and histologic findings was performed using the validated index of severity for EoE (I-SEE score). Kruskal-Wallis Rank Sum Test and Pearson’s Chi-squared were used for comparisons. Significance threshold was p<0.05.
Results:
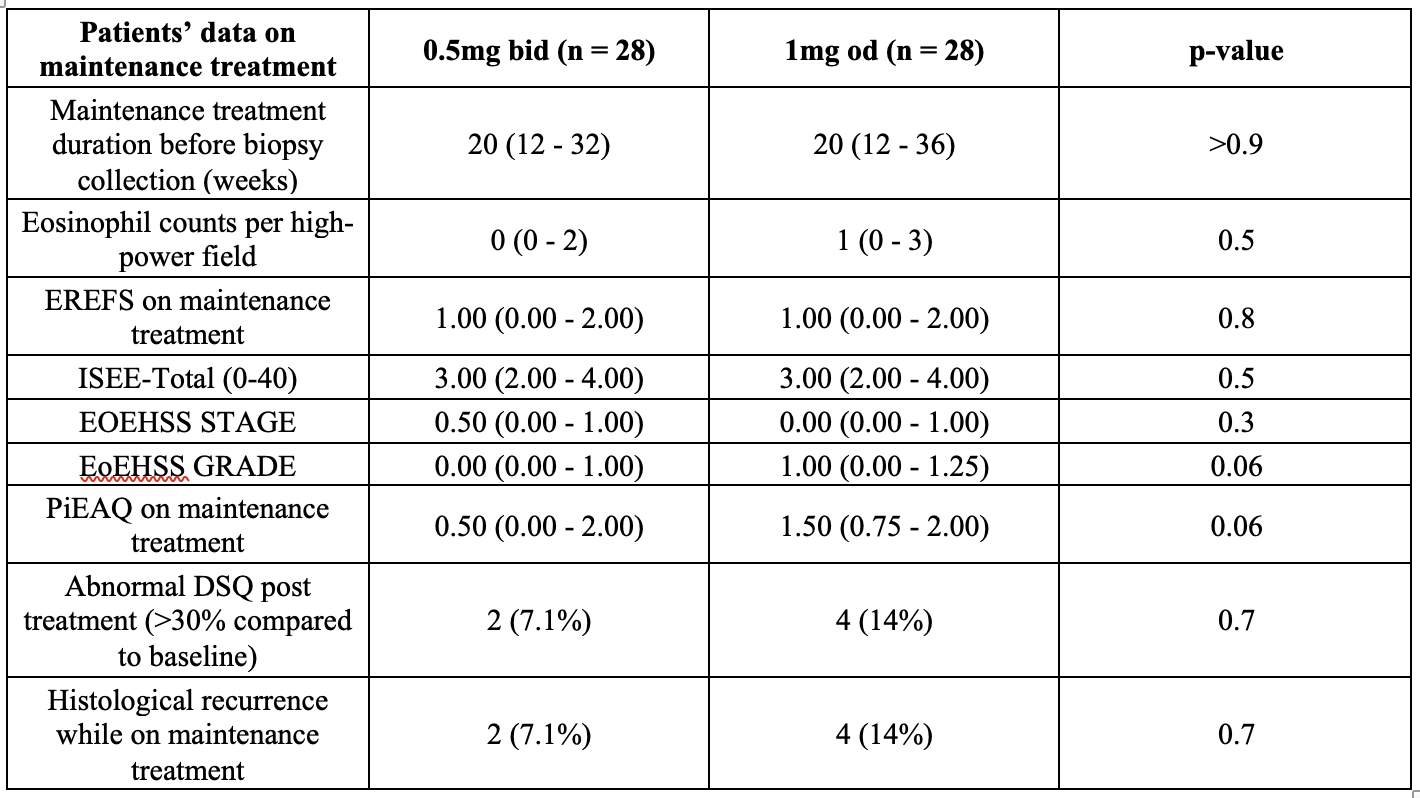
Fifty-six patients were included (46 males and 10 females). Following histological remission with BOT 1mg bid, 28 (50%) initiated maintenance treatment with BOT 1mg od and 28 (50%) with BOT 0.5 bid. At the end of induction of remission (T0), the two groups had achieved similar outcomes, with comparable median DSQ [0 (0-28) vs 0 (0-28)], EREFS [1 (0-2) vs 1 (0-1)], and PEC [1 (0-7) vs 1.5 (0-4)] (all p>0.3) (Table 1). At the end of the maintenance treatment (T1), the two groups had been taking maintenance treatment for a comparable duration [20 weeks (12 – 36) vs 20 weeks (12 – 32; p=0.7). Persistent histological and clinical remission at T1 was present in 86% of BOT 1mg od versus 93% of BOT 0.5 bid (p=0.7). In addition, EREFS scores were comparable between groups at T1 [1 (0-2) vs 1 (0-2), p=0.8]. Similarly, the EoEHSS stage and grade was comparable between groups, as was the PiEAQ score [1.5 (0.75-2) vs 0.5 (0-2); p=0.06], and the I-SEE score [ 3 (2-4) vs 3 (2-4); p=0.5] (Table 2).
Conclusions:
Persistent clinical endoscopic, and histological remission was maintained in most patients treated either with BOT 1mg od or BOT 0.5mg bid for a median of 20 weeks, indicating that the total daily dose of BOT 1mg may be more important that the frequency of administration, at least in the short term.
Table 1. Patients’ data at the end of the induction of remission period
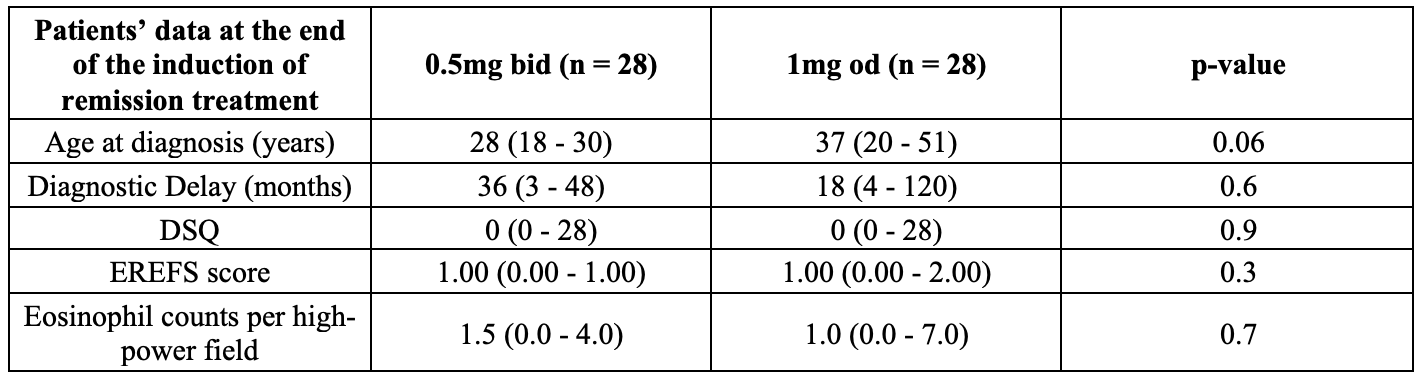
Table 2. Patients’ data at the end of the maintenance period
VALUTA ABSTRACT
Vota ogni categoria
| Originality | |
| Clarity | |
| Rationale | |
| Objectives | |
| Endpoint measures | |
| Statistics | |
| Results | |
| Relevance | |
|
Media
|
|
|
|


